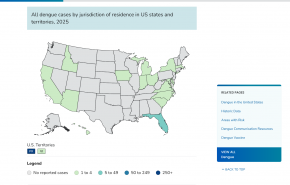
As travelers in the United States prepare for another year of record-setting Dengue fever outbreaks, a human monoclonal antibody (mAb) candidate from a Bethesda, MD-based company may soon obtain U.S. FDA approval.
As of February 12, 2025, the National Institutes of Health and AbViro LLC phase IIa clinical trial is exposing healthy volunteers in Maryland and Vermont to a weakened strain of the Dengue virus. The study will compare three AV-1 dose levels in adults challenged with DENV-3, the most infectious Dengue virus strain.
Read More